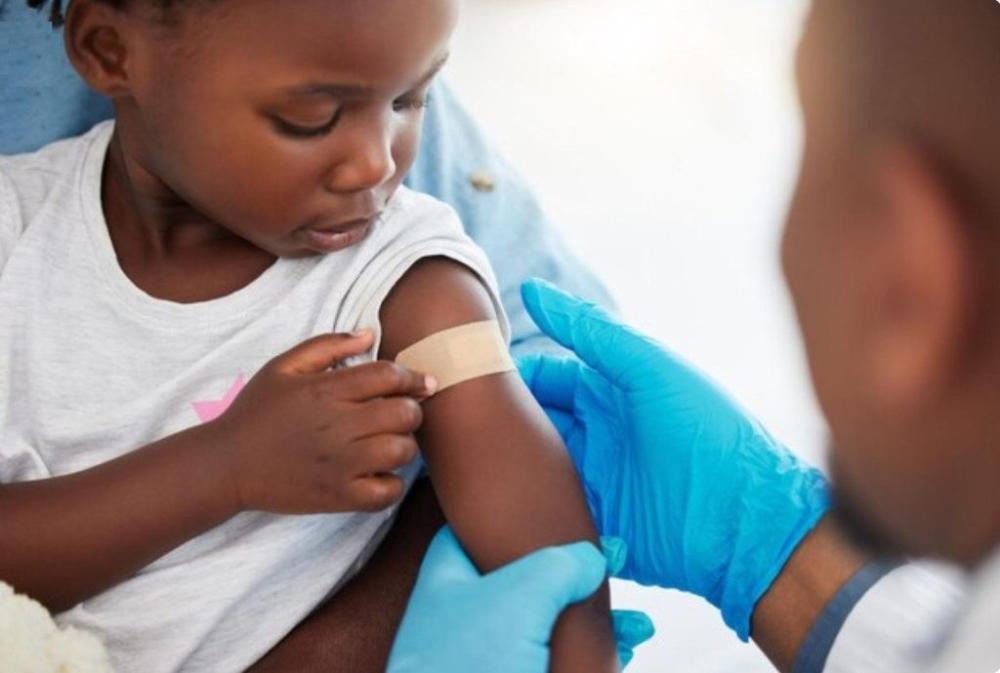
Africa-Press – Rwanda. For the first time, newborns and infants weighing less than five kilogrammes will have access to a newly approved malaria treatment, whose clinical trials were conducted in eight African countries.
The Africa Centres for Disease Control and Prevention (Africa CDC) described the approval of the new artemether-lumefantrin formulation as “a key step forward in the fight against malaria” on the continent.
No approved treatment existed for the youngest group of infants, who were often given modified doses of medicines meant for older children, an approach that carried the risk of overdose and toxicity.
The new infant-friendly formulation dissolves in breast milk and comes with a sweet flavour to make administration easier.
In a statement on August 16, Africa CDC said the treatment was developed through a partnership between Novartis and the Medicines for Malaria Venture (MMV), under the PAMAfrica consortium.
“Clinical trials were conducted in eight African Union Member States, including Burkina Faso, Côte d’Ivoire, Kenya, Malawi, Mozambique, Nigeria, Tanzania, and Uganda. The participation of these countries was instrumental in closing a longstanding gap in care for the most vulnerable.”
Swiss regulators were the first to approve the treatment, and rapid approvals are expected across the eight African countries under the Swiss agency’s Marketing Authorisation for Global Health Products procedure.
Nearly 30 million babies are born each year in malaria-endemic regions, and Novartis has indicated that the new treatment will be introduced primarily on a not-for-profit basis to increase access.
Africa CDC Director General Dr Jean Kaseya described the approval as an important advance in protecting children at the highest risk of malaria.
“The approval of the treatment is a key step forward in the fight against malaria. It ensures that even the smallest and most vulnerable infants now have access to safe and effective treatment,” he said.
Dr Ngashi Ngongo, the Principal Advisor to the Africa CDC Director General and Head of the Mpox Incident Management Support Team, added that the achievement shows the strength of Africa-led collaboration.
“The approval of the new malaria treatment demonstrates the impact of Africa-led collaboration in delivering health solutions where they are needed most,” he said.
Africa CDC confirmed that it will work with Member States to integrate the formulation into national health systems. Plans include updating clinical guidelines, training health workers on safe use, and strengthening surveillance and operational research to track safety and impact.
The agency also intends to support equitable access through local manufacturing and the African Pooled Procurement Mechanism.
Regulatory approvals will be furthered through a reliance mechanism established among the eight participating National Regulatory Authorities in collaboration with the African Medicines Regulatory Harmonization initiative, supported by the African Union Development Agency (AUDA-NEPAD).
For More News And Analysis About Rwanda Follow Africa-Press