Africa-Press – Rwanda. The pharmaceutical industry in Rwanda is set for a major boost as Labophar Ltd, the country’s oldest drug manufacturer, prepares to resume production with new facilities for intravenous (IV) fluids, syrups, and topical medicines expected to be operational by 2026.
The expansion is projected to reduce Rwanda’s heavy reliance on imported medicines, improve health security, and create a more resilient supply of essential drugs.
Meeting critical health needs
For years, the government has imported nearly all of its IV fluids, syrups, and topical treatments. IV fluids—sterile solutions administered directly into patients’ veins—are particularly vital in hospitals for hydration, nutrient delivery, and emergency care. Syrups and topical medicines also remain staples in pharmacies, especially for children and patients with chronic conditions.
Once production resumes, Labophar will manufacture 30 million IV fluid bags annually, enough to cover national demand and supply regional markets. The plant will produce 15 types of IV solutions such as normal saline, dextrose, and Ringer’s lactate, alongside cough syrups, antihistamines, multivitamins, and topical treatments including antibiotic ointments and anti-inflammatory creams.
“Our new IV facility will be state-of-the-art, using the latest German technology, and Labophar will be the first company in Sub-Saharan Africa to produce IV bags. These are now the global standard over traditional bottles,” said Pascal Gatete, Labophar’s CEO.
From shutdown to revival
Founded in 1983, Labophar had been inactive for 12 years until it was acquired in April 2024 from the Government of Rwanda by Dépôt Pharmaceutique et Matériel Médical Kalisimbi (DPMMK) Ltd. The company has since invested more than Rwf27 billion in rehabilitating and modernising the Southern Province-based facility.
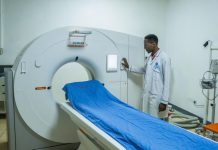
Prime Minister Justin Nsengiyumva recently visited the factory to assess progress, with management confirming that production lines for syrups, creams, and ointments will be running by early 2026, followed by tablet production later in the year.
Officials emphasise that all products will comply with international Good Manufacturing Practice (GMP) standards, ensuring quality and safety.
Reducing import dependence
The government has spent $260 million on pharmaceutical imports in the last five years. The COVID-19 pandemic further exposed the risks of relying on international supply chains, with many African countries facing shortages of critical medicines.
Labophar’s revival is seen as a strategic response. “Rwanda will go from full dependence on imported IV fluids, syrups, tablets, and topical products to self-sufficiency, with added capacity for export,” the company told The New Times.
The plant is also expected to generate more than 400 jobs, with management pledging that at least 98 percent will go to Rwandans. Positions will range from regulatory experts and quality controllers to factory floor operators.
Regional ambitions
Beyond local supply, Labophar is positioning itself as a player in the wider Sub-Saharan market. In August 2025, the company signed a joint venture and technology transfer agreement with Qatar-based Philex Pharmaceuticals to localise the production of solid dosage medicines in Rwanda.
“This agreement marks an important step in building Rwanda’s pharmaceutical manufacturing capacity and ensuring access to affordable, high-quality medicines,” said Gatete.
With Rwanda’s pharmaceutical market projected to reach $125 million in 2025, the partnership is expected to strengthen the country’s competitiveness in the region while guaranteeing local access to essential drugs.
Health security at the core
The factory’s expansion comes at a time when African nations are prioritising pharmaceutical self-sufficiency. According to health experts, the pandemic underscored the importance of local manufacturing to avoid shortages and delays.
“Our goal is to guarantee that Rwandans always have access to safe, life-saving medicines while building resilience against future health crises,” Gatete said.
If successful, Labophar’s revival will not only end Rwanda’s dependence on imported IV fluids and other critical medicines but also provide a model for how African countries can strengthen health security through domestic drug production.
Some medicines manufactured by Labophar in Huye District on September 8. Courtesy
The newly renovated facility at Labophar that will enable the firm to produce more medicines.
For More News And Analysis About Rwanda Follow Africa-Press