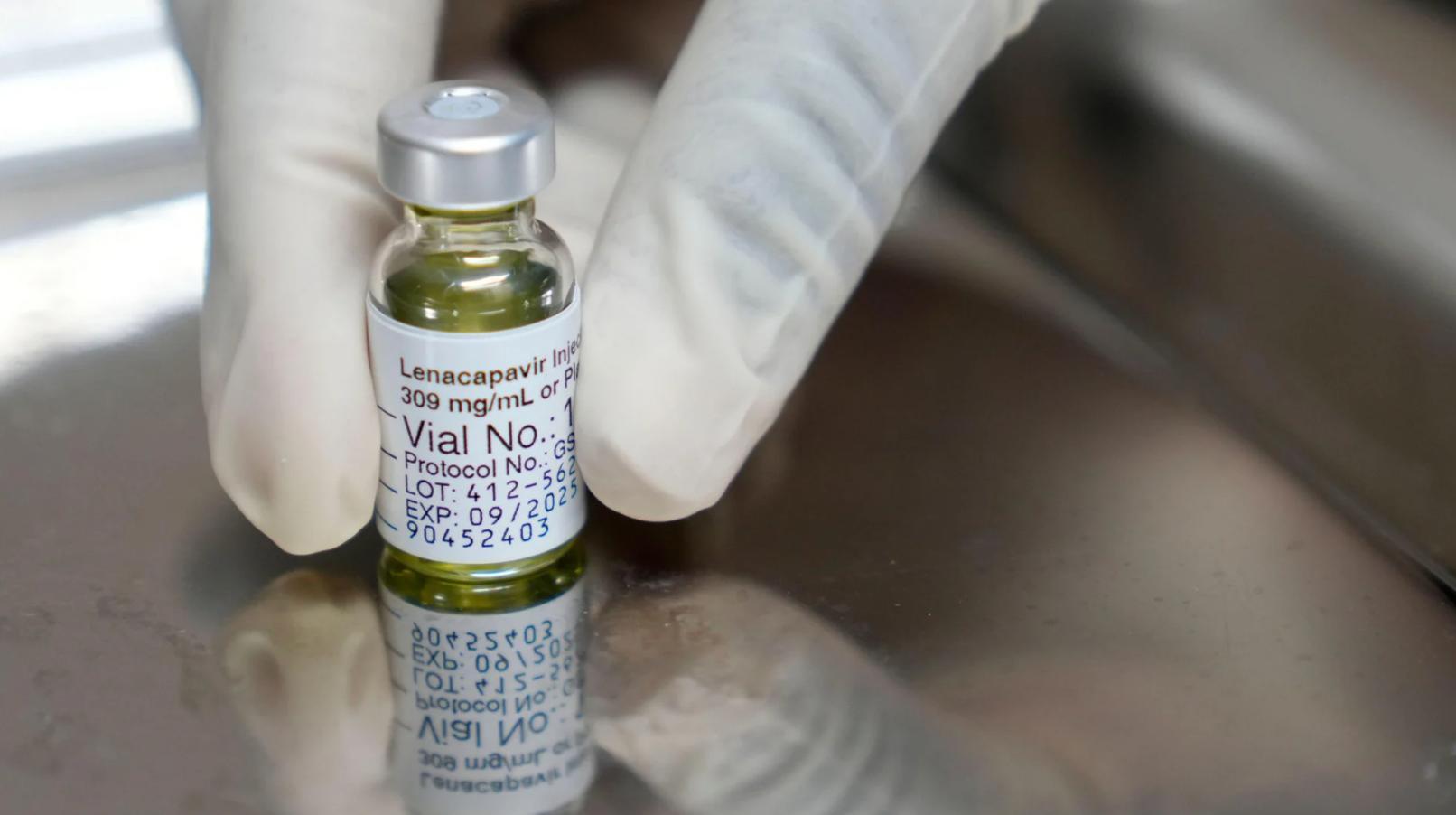
Africa-Press – Malawi. The World Health Organisation (WHO) has called for a breakthrough HIV drug, which only needs to be injected twice a year and offers near-total protection from the virus and the development of Aids, to be made available “immediately” at pharmacies, clinics and via online consultations.
Injectable lenacapavir (LEN) is a highly effective, long-acting antiretroviral alternative to daily oral pills and other shorter-acting options, according to the United Nations health agency.
GHEBREYESUS – Lenacapavir is the next best thingIn a statement issued in support of the drug and other health interventions at the 13th International Aids Society (IAS 2025) Conference, currently underway in Kigali, Rwanda, WHO Director-General Tedros Ghebreyesus said access is crucial.
“While an HIV vaccine remains elusive, lenacapavir is the next best thing: a long-acting antiretroviral shown in trials to prevent almost all HIV infections among those at risk,” Ghebreyesus said.
He added that the launch of WHO’s new guidelines on LEN, alongside the United States Food and Drug Administration’s (FDA) recent approval, marked an important step forward in expanding access to the tool.
The FDA approved the drug for use as a pre-exposure prophylaxis (PrEP) to prevent HIV infection on June 18, 2025, making it the first and only twice-yearly injectable option for HIV prevention.
“WHO is committed to working with countries and partners to ensure this innovation reaches communities as quickly and safely as possible,” Ghebreyesus said.
However, he warned that “these powerful medicines are only useful if we can get them to the people who need them”.
“It is critical to ensure that services and systems are maintained to deliver these game-changing innovations and other critical medicines,” he added.
GRINSZTEJN – Leaders must commit the funding and resourcesIAS president Beatriz Grinsztejn said the new guidelines, the licensing agreements and “promising research” signal that long-acting HIV prevention and treatment are moving closer to becoming part of everyday care.
“This is a testament to what is possible when researchers, industry, global health institutions and communities work together.
“Our next challenge is clear: leaders must commit the funding and resources needed to integrate these scientific advances into health systems quickly and equitably so that people everywhere can benefit from these life-changing options,” Grinsztejn said.
Meanwhile, advocates have also urged global leaders to ensure equitable access to new HIV prevention and treatment options.
“Long-acting HIV prevention and treatment can transform lives only if people can actually get them. We cannot repeat the mistakes of the past, where new medicines existed but stayed out of reach for the people who needed them most,” Executive Director of Advocates for the Prevention of HIV and Aids, Yvette Raphael, said.
She called for clear plans and real funding to ensure the drugs are affordable, available in clinics and trusted by communities.
RAPHAEL – Science has given us powerful tools“Science has given us powerful tools. Now we must do the work to put them in people’s hands,” Raphael said.
Data presented by Mary Mahy, Director for Data Impact at the Joint United Nations Programme on HIV/Aids, highlighted continued gaps in prevention and treatment access for key populations and regions most heavily affected by HIV.
“By the end of 2024, 73 percent of all people living with HIV had achieved viral suppression. This impressive progress ensures that people living with HIV are healthy and reduces onward HIV transmission,” Mahy said.
In his remarks, Minister of Health of Rwanda, Sabin Nsanzimana, said his country’s experience in the HIV response over the past few decades “demonstrates what is possible when countries prioritise people-centred approaches and invest in strategic partnerships”.
“Together, we met the UNAids 95-95-95 targets ahead of schedule and continue to harness cutting-edge science including long-acting medications to deliver more targeted, integrated interventions,” Nsanzimana said.
Meanwhile, a new licensing agreement between the Medicines Patent Pool and ViiV Healthcare was announced to expand access to long-acting injectable cabotegravir for HIV treatment.
“Expanding our licence with ViiV Healthcare to include long-acting cabotegravir for HIV treatment marks a significant step forward for equitable access,” Director of Strategy, Policy and Market Access at the Medicines Patent Pool, Esteban Burrone, said.
Burrone added that as the first full long-acting HIV treatment regimen now recommended by WHO, the drug answers a long-standing call from communities for an option that would maintain viral suppression without the need for daily medication.
The IAS Conference on HIV Science is considered the world’s most influential meeting on HIV research and its applications.
For More News And Analysis About Malawi Follow Africa-Press